
Dig Deeper: How Old is It? Absolute Dating
Dig Deeper is a monthly blog focusing on the basics of archaeology by taking a closer look at the exhibition Archaeology 101, which is currently featured at the Center.
Introduction
Now that we have discussed debitage, the waste left behind during tool creation, let’s dig deeper and learn about absolute dating.
Absolute Dating vs. Relative Dating
Absolute dating is the science that allows archaeologists to determine definite dates for artifacts. For example, if coins are found during an excavation, the coin is (usually) stamped with a date, and archaeologists are able to use the date stamped on the coin to determine when it was made and when it is from.
When artifacts are found that can be dated this way, other artifacts found at the same site are also dated but by using relative dating. Relative dating uses the dates of artifacts with absolute dates and infers that the other artifacts found are from around the same time and we will explore this further next month.
These types of dating techniques can be useful for archaeologists, but many times, artifacts found at sites have no dates stamped on them at all. So how do they decipher the age of an artifact like this?
Carbon-14 Dating

Another form of absolute dating is called Carbon-14 dating. This type of dating can be used to date organic artifacts. An organic artifact is an artifact that once was living, like bone or shell. Carbon is an element that is absorbed by all living things during their lifetime. A regular carbon element has an atomic weight of 12. To break it down even further, carbon is made of six protons and six neutrons. (To learn more about atoms, protons, and neutrons watch this video on YouTube.)
Carbon-14 is different from carbon in that it is an isotope of carbon. Chemical elements have one or more isotopes and these are defined as, each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons. Carbon-14 has two extra neutrons, giving it an atomic weight of 14 with six protons and eight neutrons.
So, carbon is absorbed by all living things. When a plant or animal dies, the number of carbon-14 atoms start to decline. Scientists and archaeologists know the rate of decay, which helps them to measure the remaining carbon-14 in the object and helps to determine how old it is. Carbon-14 dating can be used for organic objects that are 500 to about 50,000 years old.
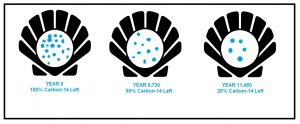
An example of this carbon-14 dating method is seen in the image to the right. After the shellfish dies, the carbon-14 atoms begin to decay. Carbon-14 has a half-life of 5,730 years, so there would be half as many carbon-14 atoms present in the shell after 5,730 years. By counting how many carbon-14 atoms remain, it can be determined when the shellfish was alive.
Up Next
We have learned about some techniques involving absolute dating, but how do archaeologists use relative dating? Come back next month to dig even deeper and learn more about relative dating!
By Jessica McPheters, Collections Manager
Learn More




